Introduction
In the present unit, firstly we shall describe various steps for effective concentration of ores. After that we shall discuss the principle of some of the common metallurgical processes. Those principles shall include the thermodynamic and electro-chemical aspects involved in the effective reduction of the concentrated ore to the metal.
Occurrence of Elements
A few elements like carbon, sulphur, gold and noble gases, occur in free state while others are found in combined forms in the earth’s crust. Elements vary in abundance. Among metals, aluminium is the most abundant. In fact, it is the third most abundant element in earth’s crust.
Metals occur in nature in free state as well as combined state. The metals which are very less reactive remain in native state. The metals which are very active occur in combined state.
1. Mineral: A natural material in which a metal or its compound occurs is called mineral.
2. Ore: A mineral from which a metal can be extracted economically is called an ore.
For example: Clay (Al2O3 SiO2.2H2O) and bauxite (Al2O3.2H2O) are two important minerals of aluminium but Al can be extracted economically from bauxite only.
Read also: The p-Block Elements Class 12 Chemistry Notes Chapter 7
Metallurgical Principles and Processes
The process of extraction of metals from their ores economically is called metallurgy. The process of extraction of each metal depends upon its nature and nature of the ore.
Important Ores and Minerals
| Metal | Ores | Composition |
| Aluminium | Corundum | Al2O3 |
| Diaspore | Al2O3.H2O | |
| Bauxite |
Al2O3.2H2O |
|
| Cryolite | Na3AlF6 |
|
| Feldspar | KAlSi3O8 | |
| IRON | Magnetite | Fe3O4 |
| Haematite | Fe2O3 | |
| Iron pyrites |
FeS2 |
|
| Chalcopyrites | CuFeS2 |
|
| Siderite | FeCO3 | |
| COPPER | Cuprite | Cu2O |
| Chalcopyrite | CuFeS2 | |
| Copper glance |
Cu2S |
|
| Malachite | CuCO3.Cu(OH)2 |
|
| ZINC | Zincite | ZnO |
| Calamine | ZnCO3 | |
| Zinc blende |
ZnS |
|
| MAGNESIUM | Magnesite | MgCO3 |
| Dolomite | MgCO3.CaCO3 | |
| Epsom salt |
MgSO4.7H2O |
|
| Carnallite | KCl.MgCl2.6H2O |
|
| Asbestos | CaMg3(SiO3)4 | |
| SILVER | Argentite or silver glance | Ag2S |
| Horn silver | AgCl | |
| LEAD | Angelsite | PbSO4 |
| Cerussite | PbCO3 | |
| TIN | Cassiterite | SnO2 |
Some Terms
(i) Matrix or Gangue: Minerals are always associated with earthy impurities known as matrix or gangue.
(ii) Flux: It is a substance used to decrease the melting point of an ore or a substance used to react with impurities to form slag.
- (a) Acidic flux: It converts basic impurities to slag. For example, SiO2 is used in the metallurgy of copper to remove FeO as FeSiO3 (slag). Other acidic fluxs are B2O3, P4O10 etc.
- (b) Basic flux: It converts acidic impurities to slag. For example, CaO is used in the metallurgy of iron to remove SiO2 as CaSiO3 (slag). Other basic fluxs are CaCO3, MgCO3. MgO etc.
(iii) Slag: The low fusible substance produced by the reaction of flux with impurities during extraction of metals, is called slag. The process is called slagging operation.
(iv) Alloy: It is a homogeneous mixture of a metal with one or more elements that may be metals or non-metals.
(v) Metallurgy: The complete scientific and technological process employed for the extraction of a metal from its ore is called metallurgy.
Read also: Electromagnetic Induction Class 12 Physics Notes Chapter 6
Extraction of Metals
The following methods used in the extraction of a metal from its ore are common in metallurgy:
- Crushing of ore
- Concentration or ore dressing
- Calcination / Roasting
- Reduction
- Purification
1. Crushing of Ores: Larger pieces of an ore are converted into small pieces by using jaw crusher and then these small pieces are converted to powder by employing (i) the stamp mill or (ii) ball mill. This process is known as pulverisation.
2. Concentration or Ore dressing: Presence of earthy matter, rock matter, sand, limestone etc in ore is called gangue or matrix. Process of removal of these impurities from ore is called concentration. Following methods are employed for concentration of ore.
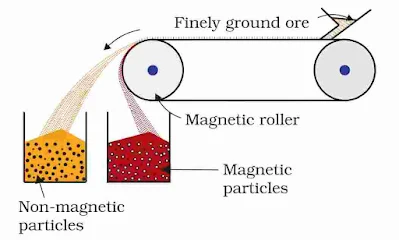
(i) Magnetic separation: This method is employed to separate the magnetic and non-magnetic components. For example, magnetic impurity of wolframite (FeWO4) is separated from tin stone (the ore of tin). Other cases are chromite ore FeO.Cr2O3, magnetite Fe3O4, pyrolusite MnO2, etc. are magnetic.
Read also: Conceptual Questions for Class 12 Physics Chapter 6 Electromagnetic Induction
(ii) Hydraulic washing (gravity separation method): This method is employed to purify heavier ore such as oxides (e.g., haematite, tin stone etc.), carbonates (e.g., calamine, malachite, etc.), native gold, etc. The ore is washed with running water using big tubs or wilfley table that has sluices (grooves). The lighter impurities are washed away and the heavier particles of the ore settle down.
(iii) Froth floatation process: This method has been in use for removing gangue from sulphide ores. In this process, a suspension of the powdered ore is made with water. To it, collectors and froth stabilisers are added. Collectors (e.g., pine oils, fatty acids, xanthates, etc.) enhance non-wettability of the mineral particles and froth stabilisers (e.g., cresols, aniline) stabilise the froth.
The mineral particles become wet by oils while the gangue particles by water. It is possible to separate two sulphide ores by adjusting proportion of oil to water or by using 'depressants'. For example, in case of an ore containing ZnS and PbS, the depressant used is NaCN. It selectively prevents ZnS from coming to the froth but allows PbS to come with the froth.
(iv) Leaching: It involves the treatment of the ore with a suitable reagent so as to make it soluble while impurities remain insoluble. The ore is recovered from the solution by suitable method. Example: Bauxite ore contains Fe2O3 and silica as impurities. When powdered ore is digested with an aqueous solution of NaOH at about 150°C under pressure, the alumina and silica dissolves forming soluble sodium meta aluminate and sodium silicate while Fe2O3 remains in the insoluble part and then Si is removed by crossing CO2.
3. (a) Calcination: It involves heating of the ore below its fusion temperature in absence of air to produce a new compound having higher % of metal as well as removing the moisture, organic matter and volatile impurities. Calcination is done in a reverberatory furnace and it makes ore porous.
Al2O3.2H2O ⟶ Al2O3 + 2H2O
(b) Roasting: It involves heating of an ore in limited supply of air below its melting point, to produce other chemical changes.
2CuFeS2 + O2 ⟶ Cu2S + 2FeS + SO2
Roasting is usually done in reverberatory furnace or blast furnace.
4. Reduction of oxide to metal
(i) Smelting (Carbon reduction method): This method can be applied for the reduction of ZnO, PbO, etc.
ZnO + C ⟶ CO + Zn
ZnO can be reduced by CO (g) above 1800°C.
Fe2O3 can be reduced by coke above 1079 K (approx) and by CO gas at a temperature lower than that.
(ii) Reduction by H2: Though, the use of H2 is expensive and not very safe, Yet H2 is used as reducing agent when carbon becomes ineffective for reduction.
WO3 + 3H2 ⟶ W + 3H2O
NiO + H2 ⟶ Ni + H2O
H2 cannot reduce Al2O3 and B2O3.
(iii) Metals as reducing agents
(a) Kroll’s process for titanium
TiCl4 + 2Mg ⟶ 2MgCl2 + Ti
(b) Gold Schmidt alumino thermite process Oxides of manganese, chromium, iron, etc. can be reduced by using aluminium powder as reducing agent. A mixture of BaO2 and Mg is used as ignition mixture to supply heat.
3Mn3O4 + 8Al ⟶ 4Al2O3 + 9Mn
(iv) Electrolytic reduction: The oxides of highly electropositive metals like Na, K, Ca, Mg etc. cannot be reduced by carbon at moderate temperature. These metals are thus obtained by the electrolysis of their oxides, hydroxides or chlorides in fused state. Sometimes, a small amount of some other salt is added to lower the fusion temperature or to increase the conductivity or both. The metal is deposited at cathode. Example: Sodium is obtained by the electrolysis of fused NaCl.
4. Refining or Purification
The metal obtained after reduction process still contains some impurities which can be removed by applying following method.
(a) Liquation process: This method is employed when impurities have higher melting point than metal. This method is used in purification of Sn, Pb and Bi etc.
(b) Distillation method: Volatile metals (Hg, Zn, Cd) are easily purified by distillation.
(c) Pyrometallurgical oxidation process: This process is used when impurities have greater affinity for oxygen than the metals itself. The oxidation is done by
- (i) Cupellation (Used to remove Pb impurity from Ag and Au etc.)
- (ii) Bessemerisation (Used in making steel from cast iron and in purification of Ni etc.)
(d) Poling: This process is used to purify the metal from its oxide. In this process, molten metal containing oxide of it as impurity is stirred by logs of green wood to reduce oxide to metal e.g CuO to Cu and SnO to Sn.
(e) Electrolytic refining: The impure metal is made anode while a thin sheet of pure metal acts as cathode. The electrolytic solution consists of an aqueous solution of salt or a complex of the metal. The soluble impurities pass into the solution and insoluble impurities collect below the anode as anode mud.
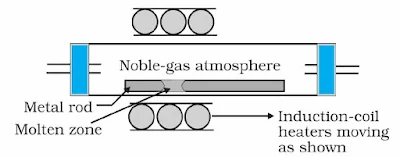
(f) Zone refining or Fractional crystallisation: This method is employed to get metals of very high purity. (Ge, Si, B, Ga, In). This method is based on the difference in solubility of impurities in molten and solid state of the metal. A movable heater is allowed to move across the impure metal rods from one end to the other end, the pure metal crystallises while the impurities pass on to the adjacent melted zone.
(g) Van Arkel process: This method is employed to obtain ultra pure metals. The impure metal is converted into a volatile compound while the impurities are not affected. The volatile compound is then decomposed electrically/thermally to get the pure metal.
Ti(s) + 2I2(g) ⟶ TiI4(g)
TiI4(g) ⟶ Ti + 2I2(g)
Other metals those can be purified by this method are Zr, V, W, Hf, Si etc.
(h) Mond’s process: This method is employed for purification of nickel. Impure nickel is converted into volatile nickel carbonyl by reaction of CO at 60-80°C. Nickel carbonyl decomposes at 180°C to form pure nickel and CO.
Ni + 4CO ⟶ Ni(CO)4 ⟶ Ni + 4CO
Extractive Metallurgy
The details of extraction process of some of the metals is as follows:
1. Extraction of Iron
Iron is extracted from its oxide ores especially from the magnetite, haematite and limonite ores. The extraction involves the following steps:
(a) Concentration of ore: The ore is concentrated by gravity separation. The washed ore is then subjected to electromagnetic separation.
(b) Calcination: Heating of ore in absence of air below melting point. Moisture and CO2 are removed.
2Fe2O3.3H2O ⟶ 2Fe2O3 + 3H2O
FeCO3 ⟶ FeO + CO2
(c) Roasting: Heating of ore in air below the melting point. Impurities (such as P, S, C, As, Sb etc.) are removed as their volatile oxides.
S + O2 ⟶ SO2
4As + 3O2 ⟶ 2As2O3
Ferrous oxide is oxidised to ferric oxide
2FeO + 1/2O2 ⟶ Fe2O3
(d) Smelting: The calcined ore is mixed with limestone (a flux) and coke (a reductant) and smelted in blast furnace.
#Blast Furnace
The smelted ore is introduced in the furnace by lowering the cup and cone arrangement and at the same time furnace is lit and a blast of hot air is sent upwards through the Tuyeres. On the basis of variation in temperature, there are four zones where different chemical changes occur.
(i) Combustion zone: This is lowest part of furnace where the temperature is about 1500°C-1600°C.
C + O2 ⟶ CO2 ΔH = – 97 kcals
CO2 + C ⟶ 2CO
(ii) Reduction zone
3Fe2O3 + CO ⟶ 2Fe3O4 + CO2
Fe3O4 + CO ⟶ 3FeO + CO2
FeO + CO ⟶ Fe + CO2
Iron formed is called sponge iron. In this reduction zone heat is also evolved due to which CO partially decomposes.
2CO ⟶ CO2 + C
(iii) Slag formation zone: This is the central zone where the temperature varies from 800-1000°C.
CaCO3 ⟶ CaO + CO2
(iv) Zone of fusion: This zone is above combustion zone. The temperature ranges between 1200-1500°C. The spongy iron melts at 1300°C and collects at the bottom of hearth. The slag being lighter floats over the molten iron and prevents oxidation of molten metal. The slag and molten metal is removed from their respective holes. The molten metals are run into moulds and are allowed to solidify.
Iron obtained from the blast furnace is called pig-iron. It contains 93% (iron), 5% (carbon) and rest (silicon, phosphorus, sulphur, manganese). The pig iron is remelted and casted into moulds. This is known as cast iron.
2. Extraction of Tin
Tin is extracted from cassiterite ore. The extraction involves the following steps:
(a) Concentration: The crushed and powdered ore is washed with running water, which removes lighter siliceous matter. Wolframite is removed by electromagnetic separator.
(b) Roasting: S and As are removed as their volatile oxides. Iron and copper pyrites are converted into their oxides and sulphates.
(c) Washing: This ore is then washed with hot water to dissolve out copper sulphate and iron sulphate. The lighter ferric oxide is washed off while the concentrated tin stone sinks to the bottom. It is called black tin.
(d) Smelting: Black tin is mixed with carbon and smelted in a reverberatory furnace. The ore is reduced to the metallic state while SiO2 is removed as slag.
SnO2 + 2C ⟶ Sn + 2CO
Molten metal is cast into ingots or blocks. It is known as block tin and contains about 99.5% of metallic tin.
(e) Purification: The metal is purified by:
- (i) Liquation: The easily fusible tin melts away and less fusible impurities (Fe, W, S, As) are left behind.
- (ii) Electrolytic refining: In this method, impure tin is made anode and a thin sheet of pure tin is made the cathode. Electrolyte consist of H2SiF6, tin sulphate and sulphuric acid. On passing current, tin is dissolved from anode and gets deposited on the cathode.
3. Extraction of Copper
The copper metal is extracted from copper pyrites. The various steps involved during extraction are:
(a) Concentration: The ore is concentrated by froth floatation process.
(b) Roasting: The concentrated ore is heated in the presence of excess of air in a reverberatory furnace. During heating temperature is kept below its melting point. Following changes are observed.
- (i) Moisture is expelled
- (ii) Sulphur, arsenic and phosphorus impurities are expelled in the form of their volatile oxides.
S + O2 ⟶ SO2
4As + 3O2 ⟶ 2As2O3
- (iii) Copper pyrite ore is converted to ferrous sulphide and cuprous sulphide
2CuFeS2 + O2 ⟶ Cu2S + 2FeS + SO2
(c) Smelting: Mixture of roasted ore, powdered coke and sand is heated in blast furnace. Following changes are observed:
- (i) Oxidation of ferrous sulphide takes place and ferrous oxide thus formed reacts with silica to form fusible slag, FeSiO3.
2FeS + 3O2 ⟶ 2FeO + 2SO2
FeO + SiO2 ⟶ FeSiO3
- (ii) Cuprous oxide which is formed as a result of oxidation is partially converted back into cuprous sulphide.
Cu2S + 3O2 ⟶ 2Cu2O + 2SO2
Cu2O + FeS ⟶ Cu2S + FeO
From the base of the furnace molten mass called matte is removed.
After the completion of reaction, the molten copper is poured off. As it cools, it gives off the sulphur dioxide dissolved in it which comes out in the form of bubbles thus giving the shape of blisters to the surface of copper, which is therefore, known as Blister copper.
(e) Purification:
- (i) Poling: The blister copper is purified by melting it in a reverberatory furnace where it is exposed to an oxidising atmosphere. The impurities are expelled as volatile oxides. During this process the molten mass is stirred with long poles of green wood. The process is known as poling. The reducing gases evolved from the wood prevent the oxidation of copper.
- (ii) Electrolytic purification: The crude copper obtained is then purified by electrolysis. Impure copper is made anode and a thin strip of pure copper is made cathode. An aqueous solution of copper sulphate, acidified with dil. H2SO4 is used as electrolyte. During electrolysis anode starts dissolving due to oxidation whereas copper cathode becomes thicker due to deposition of copper.
4. Extraction of Lead
Lead is extracted from galena (PbS) involving the following steps:
1. Concentration: The crushed, powdered ore is concentrated by the froth floatation process.
2. Reduction: The concentrated ore is reduced in two steps:
- (a) Roasting: The concentrated ore is roasted in reverberatory furnace, where the ore is partially oxidised.
2PbS + 3O2 ⟶ 2PbO + 2SO2
PbS + 2O2 ⟶ PbSO4
- (b) Smelting: After roasting, the temperature of furnace is raised and air supply is reduced. Some more concentrated galena is added. PbS reacts with PbO and PbSO4 forming lead metal.
2PbO + PbS ⟶ 3Pb + SO2
PbSO4 + PbS ⟶ 2Pb + 2SO2
Molten lead is drawn off from the lower part of the furnace.
(c) Purification: Lead obtained from furnace still contains Bi, Sb, Cu, Fe, Ag, Au, Zn, As as impurity. These impurities can be removed by the following processes.
- (i) Softening process: The impure metal is melted on the hearth of the reverberatory furnace in a current of air. The base metals are oxidised and float over the surface of the molten mass as scum, which is removed.
- (ii) Desilverisation: The removal of silver is done by Parke’s process or Pattinson’s process.
- (iii) Electrolytic refining: The desilverised lead is further purified by electrolytic method.
5. Extraction of Magnesium
Magnesium is highly electropositive and strong reducing agent. Therefore it is extracted by the electrolysis of their fused metal halides containing alkali metal halides. The function of alkali metal halides is
- (a) To lower the operating temperature.
- (b) To increase the electrical conductivity.
From Magnesite: (MgCO3)
The magnesite ore is calcinated into magnesium oxide.
MgCO3 ⟶ MgO + CO2
Magnesium from magnesium oxide is obtained either by thermal reduction or electrolytic reduction. The magnesium oxide is dissolved in a mixture of molten fluorides of magnesium, barium and sodium. The electrolysis is done by using carbon rods as anodes suspended in molten mass and cast iron rods as cathodes at 650°C. The magnesium is obtained in molten state.
6. Extraction of Zinc
1. Concentration: Powdered Zinc Blende is concentrated by froth floatation process.
2. Roasting: 2ZnS + 3O2 ⟶ 2ZnO + 2SO2
3. Reduction: ZnO + C ⟶ Zn + CO
4. Purification: Impure Zinc obtained above contain impurities of Pb, Fe, Cd, As etc. This impure Zinc is known as spelter. Firstly, Cd is distilled off at 800°C and then electrolytic purification is done using impure Zn as anode and cathode consists of sheets of pure Al. Pure Zn is scrapped off from Al-sheets.
Uses of Some Metals
Summary
Metallurgy: Sum total of processes used for the extraction of metals from their ores. It also includes their purification and alloy formation.
Ore: A mineral from which one or more metals can be extracted easily and profitably.
Flux: A substance used to reduce the m.p. of ore or react with gangue to convert it to slag.
Gangue or matrix: Earthy impurities present with minerals.
Pyrometallurgy: Method of thermal reduction (using reducing agent and heat) of ore to metal.
Hydrometallurgy: Method of extraction of metals using leaching and displacement employing cheaper and reactive metal.
Leaching: Method of reacting an ore with some reagent to collect the required metal as water soluble salt.
Ellingham diagrams: Plots of ΔfG° oxide, sulphide or halide, per mole of oxygen, sulphur or halogen respectively versus temperature.
Smelting: Heating purified oxide form of ore with coke. It may give metal or matte. It is generally known as carbon reduction method.
Aluminothermy: Method of reducing oxide of a metal by heating with powdered aluminium.