Alcohols, Phenols and Ethers Class 12 Notes Chemistry Chapter 11
Introduction
In this unit, we shall discuss the chemistry of three classes of compounds, namely — alcohols, phenols and ethers. Alcohols and phenols are formed when a hydrogen atom in a hydrocarbon, aliphatic and aromatic respectively, is replaced by –OH group. The subsitution of a hydrogen atom in a hydrocarbon by an alkoxy group (R–O/Ar–O) yields another class of compounds known as ‘ethers’.
Alcohols and Phenols : Classification
Alcohols and phenols may be classified as mono, di, tri-or polyhydric compounds depending upon number of hydroxyl groups respectively in their structures as given below:
(i) Compounds Containing Csp3 -OH bond
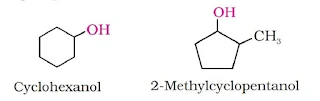
(a) Primary, secondary and tertiary alcohols: In these three types of alcohols, the –OH group is attached to 1ºC, 2ºC and 3ºC atom respectively.
Read also: Aldehydes, Ketones and Carboxylic Acids Chemistry Class 12 Notes Chapter 12
(b) Allylic alcohols: In these alcohols, the –OH group is attached to a sp3 hybridized carbon next to the carbon-carbon double bond.
(c) Benzylic alcohols: In these alcohols, the –OH group is attached to a benzylic carbon atom.
(ii) Compounds Containing Csp2 -OH bond
These alcohols contain –OH group bonded to a carbon-carbon double bond i.e., vinylic carbon or to an aryl carbon. Ex. Vinylic alcohol : CH2=CH–OH
Nomenclature
(a) Alcohols
The common name of an alcohol is derived from the common name of the alkyl group and adding the word alcohol to it.
Read also: Dual Nature of Radiation and Matter Class 12 Physics Notes Chapter 11
IUPAC name of an alcohol is derived from the name of the alkane and adding suffix ‘ol’ while the letter ‘e’ of the alkane is dropped.
CH3–OH Methane + ol = Methanol
CH3CH2–OH Ethane + ol = Ethanol
Cyclic alcohols are named using the prefix cyclo and considering the –OH group attached to C–1.
(b) Phenols
The simplest hydroxy derivative of benzene is phenol.
Dihydroxy benzene derivatives are named as 1, 2-, 1, 3- and 1, 4-benzenediol.
Read also: Conceptual Questions for Class 12 Physics Chapter 11 Dual Nature of Matter and Radiation
Alcohols and Phenols
1. Preparation of Alcohols
(i). From Alkenes
(A) By acid reflux followed by hydrolysis: Alkenes react with water in the presence of acid as catalyst. The addition reaction takes place in accordance of Markovnikov’s rule.
RCH=CH2 + H2O ⟶ RCH(OH)-CH3 + RCH2CH2OH
(B) By hydroboration-oxidation: Diborane reacts with alkenes to give trialkyl boranes, which is oxidised to alcohol by H2O2 in alkaline medium to give alcohol.
RCH=CH2 ⟶ RCH2-CH2-OH
(ii). From hydrolysis of alkyl halides
Alkyl halides upon hydrolysis yields alcohol.
RCH2-X ⟶ HO-CH2R
(iii). From carbonyl compounds
(A) By reduction of aldehyde and ketones: Aldehydes and ketones can be reduced to the corresponding alcohols by using reducing agents Pd/H2, LiAIH4, NaBH4.
R-CO-H ⟶ RCH2OH
R-CO-R ⟶ R2CH-OH
(B) By Grignard reagent: Addition of Grignard reagent to carbonyl group is the nucleophilic addition reaction. The adduct formed upon hydrolysis yields an alcohol.
R2C=O + RMgX ⟶ R2C(OH)-R
(C) From carboxylic acids: Carboxylic acids are reduced to primary alcohol in excellent yield by lithium aluminium hydride.
R-COOH + H2O ⟶ RCH2-OH
2. Preparation of Phenols
Phenols can be prepared from benzene derivatives by any of the following methods:
(i). From Haloarenes
Chlorobenzene is fused with NaOH at 623 K and 320 atmospheric pressure, phenol is obtained by acidification of sodium phenoxide.
(ii). From diazonium salt
Diazonium salts are hydrolysed to phenols by warming with water or by treating with dil.acids.
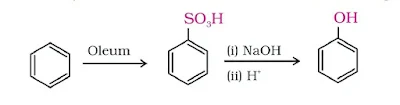
(iii). From benzene sulphonic acid
Benzene sulphonic acid is fused with NaOH. Acidification of the sodium salt gives phenol.
(iv). From cumene
Cumene is oxidised in air to form cumene hydroperoxide. It is converted to phenol and acetone by treating with dilute acid.
Physical Properties
Alcohols and phenols both are water soluble due to their ability to form H-bond with water. The solubility decreases with increase in size of hydrophobic group. Due to the same reason their boiling points are also greater than corresponding compounds of similar molecular mass except carboxylic acids. They are polar and protic in nature.
Chemical Properties
(i) Reaction with metals: Alcohol and phenol reacts with active metals such as sodium, potassium and aluminium to yield corresponding alkoxide/phenoxide and hydrogen.
CH3–CH2–OH + Na ⟶ CH3CH3–ONa + 1/2H2
(ii) Acidity of alcohols: The acidic character of alcohols is due to the polar nature of O–H bond. An electron releasing group increases electron density on oxygen tending to decrease the polarity of –OH bond. This decreases the acid strength. Hence acidic strength follows the order
(iii) Acidity of phenols: The hydroxyl group of phenol is directly attached to the sp2 hybridised carbon of benzene ring which acts as an electron withdrawing group.
(iii) Esterification: Alcohols and phenols both react with acids, acid chlorides and acid anhydrides to form esters. With carboxylic acids esterification is carried with little amounts of conc. H2SO4;
R-OH + R'COOH ⇌ R'COOR + H2O
(vi) Reaction with HX: Alcohols reacts with HX to form alkyl halides
CH3OH + HCl ⟶ CH3Cl + H2O
(vii) Reaction with PCl3 and PCl5
3R—OH + PCl3 ⟶ 3R—Cl + H3PO3
ROH + PCl5 ⟶ R — Cl + POCl3 + HCl
(viii) Reaction with SOCl2
R—OH + SOCl2 ⟶ R—Cl + SO2 + HCl
(ix) Dehydration: Alcohols undergoes dehydration to form alkene on treating with a protic acid e.g., conc. H2SO4 and H3PO4 or Al2O3.
CH3-CH(OH)-CH3 ⟶ CH3-CH=CH2 + H2O
(x) Oxidation: Oxidation state of carbon bearing – OH group is (–2) so it can be oxidised to aldehydes (Oxidation state = 0) and carboxylic acids (Oxidation state = +2) by use of suitable reagents.
RCH2–OH ⟶ RCHO ⟶ R–C(OH)=O
(xi) Nitration: With dil. HNO3 acid at low temperature phenol yields a mixture of –o and p– nitrophenols.
(xii) Halogenation: When phenol is treated with Br2 in solvents of low polarity such as CHCl3 or CS2 and at low temperature monosubstituted phenols are formed.
(xiii) Kolbe’s Reaction: Phenol when treated with NaOH it gives phenoxide ion which is more reactive than phenol, hence it undergoes electrophilic substitution reaction with CO2, a weak electrophile. Orthohydroxy benzoic acid is formed as the main product.
(xiv) Reimer-Tiemann Reaction: On treating phenol with CHCl3 in the presence of NaOH a –CHO group is introduced at o-position of benzene ring.
(xv) Reaction of phenol with zinc dust: Phenol is converted to benzene on heating with zinc dust.
(xvi) Oxidation: By application of strong oxidising agents phenol converts into benzoquinone. In the presence of air phenols are slowly oxidised to dark coloured mixtures containing quinones.
Some Commercially Important Alcohols
1. Methanol
Methanol, CH3OH, also known as ‘wood spirit’, was produced by destructive distillation of wood. Today, most of the methanol is produced by catalytic hydrogenation of carbon monoxide at high pressure and temperature and in the presence of ZnO – Cr2O3 catalyst.
CO + 2H2 ⟶ CH3-OH
Methanol is a colourless liquid and boils at 337 K. It is highly poisonous in nature. Ingestion of even small quantities of methanol can cause blindness and large quantities causes even death. Methanol is used as a solvent in paints, varnishes and chiefly for making formaldehyde.
1. Ethanol
It is obtained commercially by fermentation of sugars. The sugar is molasses.
C12H22O11 + H2O ⟶ C6H12O6 + C6H12O6
Glucose and fructose undergoes fermentation in the presence of zymase, which is found in yeast.
C6H12O6 ⟶ C2H5OH + 2CO2
In wine making, grapes are the source of sugars and yeast. As grapes ripen, the quantity of sugar increases and yeast grows on the outer skin. When grapes are crushed, sugar and the enzyme come in contact and fermentation starts. Fermentation takes place in anaerobic conditions i.e. in absence of air. Carbon dioxide is released during fermentation.
Ethers
Classification
Ethers are classified as simple or symmetrical, if the alkyl or aryl groups attached to the oxygen atom are the same, and mixed or unsymmetrical, if the two groups are different. Diethyl ether, C2H5OC2H5, is a symmetrical ether whereas C2H5-O-CH3 and C2H5-O-C6H5 are unsymmetrical ethers.
Naming of Ethers:
Common names of ethers are derived from the names of alkyl/aryl groups written as separate words in alphabetical order and adding the word ether at the end.
C2H5-O-CH3 - Ethylmethyl ether
C2H5-O-C2H5 - Diethyl ether
According to IUPAC system of nomenclature, ethers are regarded as hydrocarbon derivatives in which a hydrogen atom is replaced by an –OR or –OAr groups. The larger (R) group is chosen as the parent hydrocarbon.
C2H5-O-CH3 - Methoxyethane
C2H5-O-C2H5 - Ethoxyethane
Preparation of Ethers
1. By inter-molecular dehydration of alcohols
Alcohols undergo dehydration in presence of protic acid.
RCH2OH + HOCH2R ⟶ RCH2-O-CH2R
2. Williamson’s synthesis
It is an important method to prepare all types of ether. Alkyl halide is allowed to react with sodium alkoxide.
RO-Na + X-R ⟶ R-O-R'
Physical Properties
Due to large size of alkyl groups R–O–R bond angle in ethers is large (>110°) therefore net dipole-moment becomes less and therefore ethers show:
- (i) Low boiling points
- (ii) Low solubility in water
Solubility in water further decreases with increasing molar mass of ethers as etheral oxygen becomes more hindered and become less available to inter-molecular H-bonding with water.
Chemical Properties
(i) Cleavage of C–O bond in ethers: Ethers due to low polarity are least reactive among other functional groups. With strong acids they form alkyl halide and alcohols by “C — O” bond cleavage:
RCH2—O—CH2R + RCH2OH + RCH2—X
(ii) Electrophilic substitution: Aryl ethers show the reactions like phenols as –OR is also an activating group. The electrophilic substitution shown by aryl ethers are:
- (a) Halogenation
- (b) Friedel-Crafts Reaction
- (c) Nitration
Sammury
Alcohols and phenols are classified (i) on the basis of the number of hydroxyl groups and (ii) according to the hybridisation of the carbon atom, sp3 or sp2 to which the –OH group is attached.
Ethers are classified on the basis of groups attached to the oxygen atom.
Alcohols may be prepared (1) by hydration of alkenes (i) in presence of an acid and (ii) by hydroboration-oxidation reaction (2) from carbonyl compounds by (i) catalytic reduction and (ii) the action of Grignard reagents.
Phenols may be prepared by (1) substitution of (i) halogen atom in haloarenes and (ii) sulphonic acid group in aryl sulphonic acids, by –OH group (2) by hydrolysis of diazonium salts and (3) industrially from cumene.
Alcohols are higher boiling than other classes of compounds, namely hydrocarbons, ethers and haloalkanes of comparable molecular masses.
The ability of alcohols, phenols and ethers to form intermolecular hydrogen bonding with water makes them soluble in it.
Alcohols and phenols are acidic in nature.
Electron withdrawing groups in phenol increase its acidic strength and electron releasing groups decrease it.
Alcohols undergo nucleophilic substitution with hydrogen halides to yield alkyl halides.
Dehydration of alcohols gives alkenes.
On oxidation, primary alcohols yield aldehydes with mild oxidising agents and carboxylic acids with strong oxidising agents while secondary alcohols yield ketones.
The presence of –OH group in phenols activates the aromatic ring towards electrophilic substitution and directs the incoming group to ortho and para positions due to resonance effect.
Reimer-Tiemann reaction of phenol yields salicylaldehyde.
In presence of sodium hydroxide, phenol generates phenoxide ion which is even more reactive than phenol.
Ethers may be prepared by (i) dehydration of alcohols and (ii) Williamson synthesis.
In electrophilic substitution, the alkoxy group activates the aromatic ring and directs the incoming group to ortho and para positions.
























Join the conversation