Thermodynamics Class 11 Notes Chemistry Chapter 6
Introduction
Chemical thermodynamics deals with the relationship between various form of energy in a process. Thermodynamics deals with macroscopic properties. This chapter introduces a major subsidiary thermodynamic property, the Gibbs free energy which lets us express the spontaneity of a process in terms of the properties of the system. This chapter helps to explain why gases expand or diffuse.
System and Surrounding
(i) System : A specific portion of universe under study which is seperated from rest of the universe with a boundary is called system.
(ii) Surroundings : Rest of the universe which might be in a position to exchange energy and matter with the system is known as surrounding.
Types of System
(i) Open system : System can be open if it can exchange both energy and matter with surroundings.
(ii) Closed system : System can be closed if it can exchange energy but not matter with surroundings.
(iii) Isolated system : System can be isolated if it can neither exchange energy nor matter with surroundings.
Extensive Properties
The properties which depend upon mass of the substance is known as extensive properties i.e., mass, volume, internal energy, enthalpy etc.
Intensive Properties
The properties which are independent of mass of the substance is known as intensive properties i.e., temperature, pressure, density, refractive index.
Read also: Equilibrium Class 11 Notes Chemistry Chapter 7
Thermodynamic State of a System
A state is the condition of a system as specified by its physical properties. We can describe the state of a gas by quoting its pressure (p), volume (V), temperature (T), amount (n) etc. Variables like p, V, T are called state variables or state functions because their values depend only on the state of the system and not on how it is reached.
State Functions
The thermodynamic parameters which depends only on initial and final states of system is known as state function. i.e., internal energy(E), Enthalpy (H), entropy (S), Gibb's free energy (G).
Path Functions
The thermodynamic parameters where value does not depend merely on initial and final state but depends upon the path followed is known as path funcition. i.e., heat (q), work done (W).
Thermodynamic Process
The sequence followed to change one thermodynamic state of a system into another is called thermodynamic process. The types of thermodynamic processes are:
(a) Isothermal process: It is the process in which temperature is kept constant means temperature of initial and final state of system along with entire path of process is same.
(b) Isobaric process: It is the process in which pressure is kept constant for entire process.
(c) Isochoric process: It is the process in which volume is kept constant.
(d) Adiabatic process: The process in which heat transaction across boundary is not allowed.
Read also: Work, Energy and Power Class 11 Physics Notes Chapter 6
(e) Reversible process and Irreversible process: In thermodynamics, a process is said to be reversible when energy change in each step of the process can be reversed by changing the variables such as pressure, volume or temperature acting on them. In such a process, the driving and opposing forces differ infinitesimally and the process can be reversed completely by increasing the opposing force by an infinitesimally small amount.
Any process which does not take place in the above mentioned manner is said to be an irreversible process. In an irreversible process the driving and opposing force differ by a large amount.
(f) Cyclic process: It is the process which run in close loop means process in which initial and final states are identical.
Internal Energy
Every substance is associated with definite amount of energy that is called internal energy. It is an extensive property and a state function. Internal energy of ideal gases is a function of temperature only.
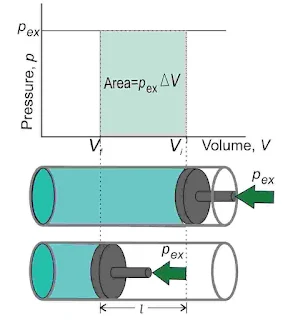
Pressure-Volume Work
It is the work done when the gas expands or contracts against the external pressure. Consider a cylinder containing one mole of an ideal gas fitted with a frictionless and weightless piston having an area of cross-section A. The total volume of the gas is Vi and the initial pressure of the gas inside P.
Read also: Conceptual Questions for Class 11 Physics Chapter 6 Work, Power and Energy
Let the external pressure acting on the piston is pex. If the external pressure Pex is slightly greater than P piston moves downward till the pressure inside the cylinder becomes equal to Pex. Let this change be achieved in a single step and the final volume be Vf. During this compression, suppose the piston moves a very small distance Δl. Thus, the work done on the gas is given by,
`w=-\int_{V_i}^{V_f}P_{ex}dV`
Heat
The change in internal energy of a system can be brought about by the transfer of heat from the surroundings to the system or vice-versa. This exchange of energy between the system and surroundings is possible as a result of the temperature difference between them. This energy called heat is represented by Q.
First Law of Thermodynamics
First law of thermodynamics states the law of conservation of energy in a different manner. According to this law, whenever a quantity of one kind of energy disappears an equivalent amount of energy appears in some other form.
According to first law of thermodynamics,
ΔQ = ∆U + ∆W
Where, Q = Heat change
W = Work done
ΔU = Change in internal energy
Enthalpy (H)
The total heat content of a system at constant pressure is known as its enthalpy. Mathematically it is the sum of internal energy and pressure-volume energy
ΔH = ΔU + PΔV
It is an extensive property and a state function. Increase in enthalpy H is equal to heat absorbed at constant pressure.
Heat Capacity
Heat capacity is amount of heat require to raise the temperature of a system by unity. It is represented as "C". It is an extensive property and temperature dependent.
Types of heat capacity
(i) Specific heat: For 1 gram system the heat loose or gained by system to bring one unit change in temperature is called specific heat denoted by 's'. specific heat is the intensive property.
`s=\frac{C}{m}`
(ii) Molar heat capacity: It is heat capacity for a system having 1 mole of material. It is represented as Cm. It is an intensive property.
`C_{m}=\frac{C}{m}`
(iii) Heat capacity at constant volume: Heat capacity of a system in isochoric condition is called heat capacity at constant volume, it is represented as Cv means molar heat capacity at constant volume.
`C_{v}=\frac{dU}{dT}`
(iv) Heat capacity at constant pressure: Heat capacity of a system in isobaric condition. It is represented by Cp means molar heat capacity at constant pressure.
`C_{p}=\frac{dH}{dT}`
Relation between Cp and Cv
We know that,
ΔH = ΔU + PΔV .....(i)
As per ideal gas equation
PΔV = RΔT .....(ii)
From (i) and (ii)
ΔH = ΔU + RΔT
`\frac{ΔH}{ΔT}=\frac{ΔU}{ΔT}+R`
`C_{p}=C_{v}+R`
`C_{p}-C_{v}=R`
Hess’s Law
According to Hess's law, If a reaction takes place in several steps then its standard reaction enthalpy is the sum of the standard enthalpies of the intermediate reactions into which the overall reaction may be divided at the same temperature.
Ist method
C(s) + O2(g) ⟶ CO2(g) = ΔH
IInd method
C(s) + 1/2O2(g) ⟶ CO(g) = ΔH1
CO(s) + 1/2O2(g) ⟶ CO2(g) = ΔH2
According to Hess's law,
ΔH = ΔH1 + ΔH2
Application of Hess's Law
- Calculation of enthalpy of formation.
- Determination of standard enthalpies of reactions.
Bond Dissociation Energy
The energy required to break one mole bond of a particular type in gaseous molecule is known as bond dissociation energy. For example, we consider the dissociation of water,
H – OH(g) ⟶ H(g) + OH(g) = ΔH = 498 kJ/mol
Entropy
Entropy is a measure of degree of randomness or disorder in a system. Entropy is an extensive property and a state function.. Its value depends upon the amount of substance present in the system.
Second Law of Thermodynamics
This states that the entropy of the universe always increases in every spontaneous (natural) change.
Free Energy (G)
Gibb's free energy is defined as,
ΔG = ΔH - TΔS
H is enthalpy, S is entropy and T is the temperature on Kelvin scale.
Summary
System : A part of universe which is under investigation.
Surroundings : The rest of the universe which is not a part of the system.
State of the system : The conditions of existence of a system when its macroscopic properties have definite values.
State functions : The thermodynamic quantities which depend only on the initial and final state of the system.
Energy is exchanged between the system and the surroundings as heat if they are at different temperatures.
The properties of the system whose value is independent of the amount of substance are called intensive properties. e.g., temperature, pressure, viscosity, surface tension, dielectric constant, specific heat capacity.
The properties of the system whose value depends upon the amount of substance present in the system are called extensive properties. e.g., mass, volume, surface area, energy, enthalpy, entropy, free energy, heat capacity.
Work is also a mode of transference of energy between system and the surroundings. Work done by the system on the surroundings is given by pΔV.
Internal energy (U) : The energy associated with the system at a particular conditions of temperature and pressure.
Enthalpy (H) : It is sum of internal energy and pressure-volume energy of the system at a particular temperature and pressure. It is also called heat content (H = E + pV).
Hess’s law : The enthalpy change in a particular reaction is the same whether the reaction takes place in one step or in a number of steps.
Bond enthalpy : The average amount of energy required to break one mole of the bonds of a particular type in gaseous molecules.
Entropy (S) : It is a measure of randomness or disorder of the system. Thus, the order is Gas > Liquid > Solid.



الانضمام إلى المحادثة