Surface Chemistry Class 12 Notes Chemistry Chapter 5
Introduction
In this chapter, we shall start with the interfacial phenomenon and its significance. We shall define adsorption and its classification and explain its mechanism, with adsorption isotherms. We will also discuss the catalysis, types of catalysis and catalysts and also describe the theories of catalysis. Thereafter we will enumerate the nature of colloidal state, describe its preparation, properties and purification of colloids.
Surface chemistry
Surface chemistry is the branch of chemistry that deals with the study of phenomenon occurring at the surface or interface, i.e., at the boundary separating two bulk phases.
Adsorption
The phenomenon of attracting and retaining the molecules of a substance by a solid (or a liquid) on its surface resulting into a higher concentration of the molecules on the surface is known as adsorption. Ex- Water vapours are absorbed by the silica gel, Ammonia is adsorbed by charcoal.
Some Basic Definitions
ii). Adsorbent
The solid on whose surface molecules of gas, vapour or solute are adsorbed is known as adsorbent.
iii). Adsorbate
The gas or solute which is adsorbed on the surface of solid is called adsorbate.
iv). Absorption
The phenomenon in which a substance is uniformly distributed throughout the body of a solid or a liquid is known as absorption. Ex- Water vapours are absorbed by the anhydrous calcium chloride.
v). Sorption
When both adsorption and absorption process takes place such that concentration of adsorbate is not same on the surface and in bulk, then common term sorption is used.
vi). Desorption
The process in which adsorbate is removed from the adsorbent is known as desorption.
Read also: General Principles and Processes of Isolation of Elements Class 12 Chemistry Notes Chapter 6
Types of Adsorption
Adsorption can be classified in two ways :
Physisorption: It is the adsorption in which weak van der Waals forces exist between adsorbate and adsorbent.
Chemisorption: It is the adsorption in which adsorbate are held to adsorbent by strong forces (as strong as chemical bond).
| S.No. | Physisorption | Chemisorption |
| 1. |
It arises because of van der Waals’ forces. | It is caused by chemical bond formation. |
| 2. |
It is not specific in nature. |
It is highly specific in nature. |
| 3. |
It is reversible in nature. | It is irreversible. |
| 4. |
It depends on the nature of gas. More easily liquefiable gases are adsorbed readily. |
It also depends on the nature of gas. Gases which can react with the adsorbent show chemisorption. |
| 5. |
Enthalpy of adsorption is low (20-40 kJ mol)in this case. | Enthalpy of adsorption is high (80-240 kJ mol) in this case. |
| 6. |
Low temperature is favourable for adsorption. It decreases with increase of temperature. | High temperature is favourable for adsorption. It increases with the increase of temperature. |
| 7. |
No appreciable activation energy is needed. | High activation energy is sometimes needed. |
| 8. |
It depends on the surface area. It increases with an increase of surface area. | It also depends on the surface area. It too increases with an increase of surface area. |
Read also: Magnetism and Matter Class 12 Physics Notes Chapter 5
Factors which affect Adsorption
(i). Temperature
Adsorption is an exothermic process. According to Le Chatelier’s principle, adsorption increases with decrease in temperature.
Adsorption isobar: The curve showing the effect of temperature on the extent of adsorption (at a given pressure) is called an adsorption isobar. (P = constant)
(ii). Pressure
With increase in pressure, adsorption also increases. This can be explained on the basis of Le Chatelier’s principle.
Adsorption isotherm: The curve showing the effect of pressure on the adsorption (at a given temperature) is called adsorption isotherm.
Freundlich adsorption isotherm
`\frac{x}{m}\propto p^{1/n}`
`\frac{x}{m}=Kp^{1/n}`
`log\frac{x}{m}=log K+\frac{1}{n}log P`
Here, `x/m` is the amount of adsorbate adsorbed per gram adsorbent.
(iii). Nature of adsorbate and adsorben
Larger the surface area of adsorbent greater is the extent of adsorption. The surface area per gram of the adsorbent is called specific surface area of adsorbent.
Read also: Conceptual Questions for Class 12 Physics Chapter 5 Magnetism and Matter
Applications of Adsorption
Production of high vacuum: The remaining traces of air can be adsorbed by charcoal from a vessel evacuated by a vacuum pump to give a very high vacuum.
Gas masks: Gas mask (a device which consists of activated charcoal or mixture of adsorbents) is usually used for breathing in coal mines to adsorb poisonous gases.
Control of humidity: Silica and aluminium gels are used as adsorbents for removing moisture and controlling humidity.
Removal of colouring matter from solutions: Animal charcoal removes colours of solution by adsorbing coloured impurities.
Separation of inert gases: Due to the difference in degree of adsorption of gases by charcoal, a mixture of noble gases can be separated by adsorption on coconut charcoal at different temperatures.
In curing diseases: A number of drugs are used to kill germs by getting adsorbed on them.
Froth floatation process: A low grade sulphide ore is concentrated by separating it from silica and other earthy matter by this method using pine oil and frothing agent.
Adsorption indicators: Surfaces of certain precipitates such as silver halides have the property of adsorbing some dyes like eosin, fluorescein, etc. and thereby producing a characteristic colour at the end point.
Catalysis
The Substances, which alter the rate of a chemical reaction and themselves remaining chemically and quantitatively unchanged after the reaction is called catalyst.
Promoters and Poisons
Promoters are substances that enhance the activity of a catalyst while poisons decrease the activity of a catalyst. For example, in Haber’s process for manufacture of ammonia, molybdenum acts as a promotor for iron which is used as a catalyst.
N2(g) + 3H2(g) ⟶ 2NH3(g)
Types of Catalysis
(i). Homogeneous Catalysis
When the reactants and the catalyst are in the same phase (i.e., liquid or gas), the process is said to be homogeneous catalysis. The following are some of the examples of homogeneous catalysis.
Ex- Hydrolysis of methyl acetate is catalysed by H+ ions furnished by hydrochloric acid.
CH3COOC2H5(l) + H2O(l) ⟶ CH3COOH(aq) + C2H5OH(aq) (catalyst = HCl)
Both the reactants and the catalyst are in the same phase.
(ii). Heterogeneous Catalysis
The catalytic process in which the reactants and the catalyst are in different phases is known as heterogeneous catalyst.
Ex- Combination between dinitrogen and dihydrogen to form ammonia in the presence of finely divided iron in Haber’s process.
N2(g) + 3H2(g) ⟶ 2NH3(g) (catalyst = Fe)
The reactants are in gaseous state while the catalyst in the solid state.
Types of Catalysis
(i). Positive Catalyst
When the rate of reaction is accelerated by a foreign substance, it is said to be a positive catalyst and the phenomenon is positive catalysis.
2H2O2(l) ⟶ 2H2O(l) + O2(g) (catalyst = Pt)
(ii). Negative catalyst
There are certain substances which, when added to the reaction mixture, retards the reaction rate instead of increasing it. These are called negative catalysts (Inhibitors) and the phenomenon is known as negative catalysis. Ex- Tetraethyl lead acts as an antiknocking agent in case of petrol. Thus, it decreases knocking of petrol and acts as a negative catalyst.
(iii). Auto-catalyst
In certain reactions, one of the product acts as a catalyst. In the initial stages the reaction is slow, but as soon as the products come into existance, the reaction rate increases. This product acting as a catalyst is auto-catalyst and the phenomenon is called auto-catalysis.
CH3COOC2H5(l) + H2O(l) ⟶ CH3COOH(aq) + C2H5OH(aq)
(Acetic acid acts as auto-catalyst)
(iv). Induced-catalyst
When the reaction influences the rate of other reaction, which does not occur under ordinary conditions, it acts as induced catalyst and the phenomenon is induced catalysts. Ex- If oxalic acid is added to a mixture of KMnO4 and HgCl2, both reduces simultaneously. Thus, reduction of KMnO4 induces the reduction of HgCl2.
Characteristics of Catalysis
- Catalyst remains unchanged in mass and chemical composition at the end.
- The catalyst cannot initiate the reaction.
- Catalyst cannot change the equilibrium position.
- Promotors (activators) are substances mixed with catalyst to increase its efficiency.
- A catalyst has a particular temperature at which its activity is maximum. This is optimum temperature.
Enzyme Catalysis
Enzymes are complex heterogeneous organic compounds which are produced by living plants and animals. They are actually protein molecules of high molecular mass and form colloidal solutions in water. They are very effective catalysts, catalyse numerous reactions, especially those connected with natural processes.
Numerous reactions that occur in the bodies of animals and plants to maintain the life process are catalysed by enzymes. The enzymes are, thus termed as biochemical catalysts and the phenomenon is known as biochemical catalysis.
(i). Inversion of cane sugar: The invertase enzyme converts cane sugar into glucose and fructose.
C12H22O11(aq) + H2O(l) ⟶ C6H12O6(aq) + C6H12O6(aq)
(ii). Conversion of glucose into ethyl alcohol: The zymase enzyme converts glucose into ethyl alcohol and carbon dioxide.
C6H12O6(aq) ⟶ C2H5OH(aq) + CO2(g)
(iii). In stomach, the pepsin enzyme converts proteins into peptides while in intestine, the pancreatic trypsin converts proteins into amino acids by hydrolysis.
(iv). Conversion of milk into curd: It is an enzymatic reaction brought about by lacto bacilli enzyme present in curd.
Colloidal System
A colloid is a heterogeneous system in which one substance is dispersed as very fine particles in another substance called dispersion medium. Colloidal particles are larger than simple molecules but small enough to remain suspended. Their range of diameters is between 1 and 1000 nm (10–9 to 10–6 m).
Classification of Colloids
(i). Classification based on physical states of dispersed phase and dispersion medium: Dispersed phase may be a solid, liquid or a gas. Dispersion medium can also be a solid, a liquid or a gas.
(ii). Classification based on the nature of interaction between dispersed phase and dispersion medium:
a). Lyophilic colloids: Substances like protein, starch, rubber, whose molecules pass readily into colloidal state, whenever mixed with a suitable solvent. Such colloids are known as lyophilic colloids.
b). Lyophobic colloids: Substances such as arsenic sulphide, gold and other metals which are sparingly soluble. These substances do not pass into colloidal state readily and are called lyophobic colloids.
(iii). Classification based on type of particles of dispersed phase:
a). Multimolecular colloids: On dissolution, a large number of atoms or smaller molecules of a substance aggregate together to form species having size in colloidal range (diameter < 1 nm) Example : Gold sol, sulphur sol
b). Macromolecular colloids: They are formed when macromolecules added in suitable solvent for solution in which the size of macromolecules in the colloidal range. Such colloids are quite stable and resembles true solution in many respects. Example : Starch, cellulose, proteins and enzymes are natural macromolecular colloids but polythene, nylon, polystyrene, synthetic rubber etc. are man-made colloids.
c). Associated colloids (Micelles): The substances which when dissolved in a medium at low concentration behave as normal strong electrolyte but at higher concentration exhibit colloidal behaviour due to formation of aggregates. The aggregates particle is called micelles. The concentration above which micelles can be formed called critical micelles concentration (CMC) and temperature above which micelles can be formed called Kraft temperature. Example: Synthetic detergent and soaps.
Mechanism of micelles formation
When soap dissolve in water it dissociates into RCOO– & Na+. RCOO– ions consist of two parts i.e., non-polar part R (called tail) which is water repelling and polar part COO– (called head) which is water loving. The RCOO– ion present on the surface with their COO– groups in water and R group staying away from water. But at the CMC, anions are pulled into the bulk of the solution and aggregate to form a spherical shape with their hydrocarbons chain pointing towards centre of the sphere with COO– part remaining outward on the surface of the sphere. An aggregate thus formed called ionic micelles. These micelles may contain as many as 100 such ions.
Some Important Term
Peptization
Certain freshly formed precipitates of silver chloride, aluminium hydroxide can be converted into colloidal solution by the addition of small amount of suitable electrolyte having one common ion. This process is called peptization.
Coagulation or Precipitation
The coagulation of colloids is due to neutralisation of charge due to which the particles come nearer to each other to form aggregates and settle down under the force of gravity. The process of setting of colloidal particles is called coagulation of the sol.
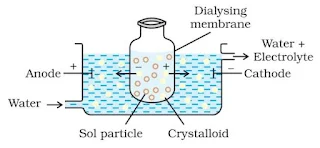
Dialysis
It is a process of removing dissolved substance from a colloidal solution by means of diffusion through a suitable membrane. The apparatus used for this purpose is called dialyser.
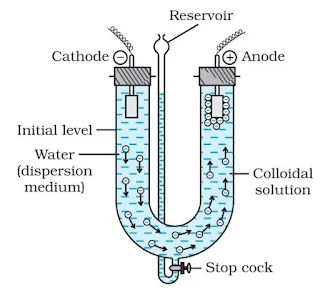
Electro-dialysis
Dialysis is a slow process and can be made faster by applying an electric field if the impurity is only an electrolyte, this process is called electro-dialysis.
Ultrafiltration
An ultrafilter paper may be prepared by soaking the filter paper in a colloidion solution hardening by formaldehyde and then finally drying. Ultra filter paper allows to pass electrolyte particles present as impurity but not allow to pass colloidal particles. Ultrafiltration is a slow process, to speed up the process, pressure or suction is applied. The colloidal particles on stirring with fresh dispersion medium to get pure colloidal solution.
Tyndall effect
When a beam of light strikes the surface of colloidal particles then path of beam get illuminated due to scattering of light by colloidal particles, this phenomenon is called tyndall effect. This effect was first observed by Faraday and latter studied in details by Tyndall. The bright cone of the light is called tyndall cone.
Brownian movement
British Botanist Robert Brown observed that colloidal particles are in continuous zig-zag motion when viewed under a powerful ultramicroscope called Brownian movement. This motion is independent of the nature of colloids but depends on the size of particles and viscosity of the solution.
Electrophoresis
The movement of colloidal particles under the applied electric potential is called electrophoresis. Positively charged particles move towards the cathode while negatively charged particles move towards the anode when electric potential is applied across two platinum electrodes dipping in a colloidal solution.
Hardy-Schulze rule
According to this rule, "Higher the valence of the flocculating ions added, the greater is its power to cause precipitation." Coagulating power for negatively charged solution : Al3+ > Ba2+ > Na+
Protection of colloids
In order to prevent the lyophobic colloids from coagulation, some suitable lyophilic colloid is added to it before the addition of electrolyte is called protection of colloids. Due to addition of lyophilic colloids a layer of it is formed over the lyophobic colloids due to adsorption so no direct contact between lyophobic colloids and electrolytes.
The lyophilic colloid added is called protective/protecting colloids and lyophobic colloids is called protected colloids. The protective power of a lyophilic colloids is measured in terms of gold number which is defined as the number of milligrams of the protective colloids required to just prevent the coagulation of 10 ml of standard gold sol when 1 ml of 10% solution of NaCl is added to it is called gold number. Smaller the value of gold number, greater will be protective power of the lyophilic colloid.
Emulsions
These are liquid-liquid colloidal systems, i.e., the dispersion of finely divided droplets in another liquid. If a mixture of two immiscible or partially miscible liquids is shaken, a coarse dispersion of one liquid in the other is obtained which is called emulsion. There are two types of emulsions.
- Oil dispersed in water (O/W type) and
- Water dispersed in oil (W/O type).
In the first system, water acts as dispersion medium. Examples of this type of emulsion are milk and vanishing cream. In milk, liquid fat is dispersed in water. In the second system, oil acts as dispersion medium. Common examples of this type are butter and cream.
Application of colloids
Electrical precipitation of smoke: The smoke before it comes out from the chimney, is led though a chamber containing plates having a charge opposite to that carried by smoke particles and on coming in contact with these plates lose their charge and get precipitated. The precipitator is called Cottrell precipitator.
Purification of drinking water: It is done by adding alum in water.
Medicines: Colloidal antimony is used in curing kalaazar, argyrol is silver sol used as an eye lotion, colloidal gold is used for intramuscular injection, milk of magnesia, an emulsion is used for stomach disorder etc.
Tanning of leather.
Cleansing action of soaps and detergent by micelles formation.
Photographic plates and films are prepared by coating an emulsion of light-sensitive silver bromide in gelation over glass plates or celluloids films.
Rubber industry: Rubber is obtained by coagulation of latex.
Industrial products like paints, inks, synthetic plastics, rubber, graphite lubricants, cement etc. are all colloidal solutions.
Summary
Surface chemistry deals with the phenomena that occur at the surfaces or interfaces.
Adsorption is the phenomenon of attracting and retaining the molecules of a substance on the surface of a solid resulting into a higher concentration on the surface, than in the bulk.
In physisorption, adsorbate is held by adsorbent by weak van der Waal’s forces, and in chemisorption, adsorbate is held by the adsorbent by strong chemical bond.
Almost all solids adsorb gases. The extent of adsorption of a gas on a solid depends upon nature of gas, nature of solid pressure of gas and temperature of gas.
The relationship between the extent of adsorption and pressure of the gas at constant temperature is known as adsorption isotherm.
Colloidal solutions are intermediate between true solutions and suspensions. A colloidal system consists of two phase: dispersed phase and the dispersion medium.
The colloidal system shows interesting optical, mechanical and electrical properties.
The process of charging the colloidal particles in a sol into the insoluble precipitate by addition of some suitable electrolytes is known as coagulation.
Catalyst is a substance which enhances the rate of a chemical reaction without itself getting used up in the reaction. The phenomenon of using catalyst is known as catalysis.
Higher the charge on ions, lesser the coagulation value for the precipitation of oppositely charged colloids.
The amount of protective colloids required to prevent the change of colour of 100 ml of 0.01% of Congo Rubin dye by the addition of 0.16 equivalent of KCl is called Congo Rubin number.
Isoelectric point: pH above which sol is negatively charged and below which positively charged and at that pH uncharged is called isoelectric point of colloids. At isoelectric point, the colloidal particles do not migrate under the influence of electric field.
The colloidal sol of cellulose nitrate in ethyl alcohol is called colloidion.
The existance of electroosmosis has suggested that when liquid is forced through a porous material or a capillary tube, a potential difference is set up between two sides called as streaming potential.
The sedimentation potential is set up when a particle is forced to make in resting liquid.










Join the conversation