The p-Block Elements Class 12 Notes Chemistry Chapter 7
Introduction
In periodic table, p-block elements are placed in Group 13 to Group 18, having general valence shell electronic configuration ns2 np1–6. Properties of p-block elements are influenced by variation in atomic size, ionization energy, electron gain enthalpy and electronegativity. The 1st element of each group shows anomalous behaviour from rest of members of same group.
Group 15 Elements
Group 15 includes nitrogen, phosphorus, arsenic, antimony, bismuth and moscovium. As we go down the group, there is a shift from non-metallic to metallic through metalloidic character. Nitrogen and phosphorus are non-metals, arsenic and antimony metalloids, bismuth and moscovium are typical metals.
Properties of Group 15
1. Electronic Configuration
The valence shell electronic configuration of these elements is ns2np3. The s orbital in these elements is completely filled and p orbitals are half-filled.
2. Atomic and Ionic Radii
Covalent and ionic radii increase in size down the group. There is a considerable increase in covalent radius from N to P.
3. Ionisation Enthalpy
Down the group, ionisation enthalpy decreases due to gradual increase in atomic size. Also ionization enthalpy of Group 15 elements much higher than that Group 14 element in a particular period.
4. Electronegativity
Down the group electronegativity value decreases, but difference in value is not much in heavier elements.
5. Physical State
Nitrogen is a diatomic gas, while other elements are polyatomic solid. Boiling point increases down the group, but that of Sb is greater than Bi. Melting point increases upto arsenic and then decreases upto bismuth. All elements of group 15 show allotropy except nitrogen.
Read also: The d and f-Block Elements Chemistry Class 12 Notes Chapter 8
6. Chemical Properties
Oxidation State : Common oxidation state of Group 15 elements are –3, +3 and +5. Tendency to show –3 oxidation state decreases down the group due to increase in size and metallic character. Bi hardly forms any compound in –3 oxidation state. The stability of +5 oxidation state decreases and that of +3 oxidation state increases down the group due to inert pair effect. Nitrogen shows +1, +2, +4 oxidation state also when it reacts with oxygen.
Covalency : Nitrogen shows maximum covalency of four, as only four orbitals (one s and three p) are available for bonding. The heavier element with vacant d orbital can show covalency more than four. All Group 15 elements form hydrides of the type EH3. All Group 15 elements form oxides of type E2O3 and E2O5. The oxide in higher oxidation state is more acidic than in lower oxidation state. Elements of group 15 reacts to form halide of type EX3 and EX5. All these elements react with metals to form their binary compounds exhibiting –3 oxidation state, such as, Ca3N2 (calcium nitride).
Anomalous properties of nitrogen
Nitrogen differs from the rest of the members of 15 group due to its small size, high electronegativity, high ionisation enthalpy and non-availability of d orbitals. Nitrogen has unique ability to form pπ-pπ multiple bonds with itself and with other elements having small size and high electronegativity. nitrogen exists as a diatomic molecule with a triple bond (one s and two p) between the two atoms. Consequently, its bond enthalpy (941.4 kJ mol–1) is very high.
Dinitrogen
Preparation
Dinitrogen is produced commercially by the liquefaction and fractional distillation of air. Liquid dinitrogen (b.p. 77.2 K) distils out first leaving behind liquid oxygen (b.p. 90 K).
In the laboratory, dinitrogen is prepared by treating an aqueous solution of ammonium chloride with sodium nitrite.
NH4Cl(aq) + NaNO2(aq) ⟶ N2(g) + 2H2O(l) + NaCl(aq)
Read also: Alternating Current Class 12 Physics Notes Chapter 7
Properties
It is colourless, odourless, tasteless and nontoxic gas. It has two stable isotopes N14 and N15.
It has very low solubility in water (23.2 cm3 per litre of water at 273 K and 1 bar P) and low freezing and boiling point.
Dinitrogen is inert at room temperature because of high bond enthalpy of N ☰ N bond. With increase in temperature reactivity increases.
It combines with H2 at about 773 K in presence of catalyst (Haber’s process) to form ammonia.
N2(g) + 3H2(g) ⟶ 2NH3(g)
Ammonia
Preparation
Ammonia is present in small quantities in air and soil, formed by decay of nitrogenous organic compounds e.g., urea.
NH2CONH2 + 2H2O ⟶ (NH4)2CO3 ⟶ 2NH3 + H2O + CO2
On small scale, it is obtained from ammonium salts which decompose when treated with caustic soda or lime.
2NH4Cl + Ca(OH)2 ⟶ 2NH3 + 2H2O + CaCl2
(NH4)2SO4 + 2NaOH ⟶ 2NH3 + 2H2O + Na2SO4
Structure
Read also: Conceptual Questions for Class 12 Physics Chapter 7 Alternating Current
Properties
Ammonia is colourless gas with pungent odour. Freezing point = 198.4 K. Boiling point = 239.7 K. As it is associated through hydrogen bonding in solid and liquid states it has higher melting and boiling point, than expected on the basis of its molecular mass.
Ammonia is highly soluble in water. Its aqueous solution is weakly basic due to formation of OH– ions.
NH3(g) + H2O(l) ⟶ NH4+(aq) + OH–(aq)
As a weak Lewis base, it precipitates hydroxide of many metals from their salt solutions e.g.,
ZnSO4 + 2NH4OH ⟶ Zn(OH)2 + (NH4)2SO4
Oxides of Nitrogen
Nitric Acid
Preparation
In laboratory HNO3 prepared by heating KNO3 or NaNO3 and conc. H2SO4 in glass retort.
NaNO3 + H2SO4 ⟶ NaHSO4 + HNO3
Structure
Properties
- It is colourless liquid.
- Freezing point is 231.4 K and boiling point is 355.6 K.
- HNO3 contains ~68% of HNO3 by mass and has specific gravity 1.504.
- In aqueous solution, HNO3 behaves as strong acid giving hydronium and nitrate ions.
- Concentrated nitric acid is strong oxidising agent and attacks most metals except noble metals such as gold and platinum.
Brown Ring Test for Nitrates
This test is done by adding dil. FeSO4 to an aqueous solution containing NO3- , and then adding conc. H2SO4 along the sides of test tube. Brown ring at interface between solution and H2SO4 layer indicates the presence of NO3- in solution.
NO3- + 3Fe2+ + 4H+ ⟶ NO + 3Fe3+ 2H2O
[Fe(H2O)6]2+ + NO ⟶ [Fe(H2O)5(NO)]2+ + H2O
Allotropic Forms of Phosphorus
Phosphorus is found in many allotropic forms, the important ones being white, red and black.
White phosphorus is a translucent white waxy solid. It is poisonous, insoluble in water but soluble in carbon disulphide and glows in dark. It dissolves in boiling NaOH solution in an inert atmosphere giving PH3.
P4 + NaOH + 3H2O ⟶ PH3 + 3NaH2PO2
Red phosphorus is obtained by heating white phosphorus at 573K in an inert atmosphere for several days. Red phosphorus possesses iron grey lustre. It is odourless, non-poisonous and insoluble in water as well as in carbon disulphide. Chemically, red phosphorus is much less reactive than white phosphorus. It does not glow in the dark.
Black phosphorus has two forms α-black phosphorus and β-black phosphorus. α-Black phosphorus is formed when red phosphorus is heated in a sealed tube at 803K. β-Black phosphorus is prepared by heating white phosphorus at 473 K under high pressure.
Phosphine (PH3)
Preparation
Phosphine is prepared by the reaction of calcium phosphide with water or dilute HCl.
Ca3P2 + 6H2O ⟶ 3Ca(OH)2 + 2PH3
Properties
It is a colourless gas with rotten fish smell and is highly poisonous. It explodes in contact with traces of oxidising agents like HNO3, Cl2 and Br2 vapours. It is slightly soluble in water. The solution of PH3 in water decomposes in presence of light giving red phosphorus and H2.
3CuSO4 + 2PH3 ⟶ Cu3P2 + 3H2SO4
Uses
The spontaneous combustion of phosphine is technically used in Holme’s signals. Containers containing calcium carbide and calcium phosphide are pierced and thrown in the sea when the gases evolved burn and serve as a signal. It is also used in smoke screens.
Phosphorus Halide
Phosphorus forms two types of halides, PX3 (X = F, Cl, Br, I) and PX5 (X = F, Cl, Br).
(i). Phosphorus Trichloride
Preparation
It is obtained by passing dry chlorine over heated white phosphorus.
P4 + 6Cl2 ⟶ 4PCl3
Properties
It is a colourless oily liquid and hydrolyses in the presence of moisture.
PCl3 + 3H2O ⟶ H3PO3 + 3HCl
(ii). Phosphorus Pentachloride
Preparation
Phosphorus pentachloride is prepared by the reaction of white phosphorus with excess of dry chlorine.
P4 + 10Cl2 ⟶ 4PCl5
Properties
PCl5 is a yellowish white powder and in moist air, it hydrolyses to POCl3 and finally gets converted to phosphoric acid.
PCl5 + H2O ⟶ POCl3 + 2HCl
When heated, it sublimes but decomposes on stronger heating.
PCl5 ⟶ PCl3 + Cl2
Oxoacids of Phosphorus
Phosphorus forms a number of oxoacids. The important oxoacids of phosphorus with their formulas given in Table.
The compositions of the oxoacids are interrelated in terms of loss or gain of H2O molecule or O-atom. The structures of some important oxoacids are given below :
Group 16 Elements
Oxygen, sulphur, selenium, tellurium, polonium and livermorium constitute Group 16 of the periodic table. This is sometimes known as group of chalcogens. The name is derived from the Greek word for brass and points to the association of sulphur and its congeners with copper.
Properties of Group 16
1. Electronic Configuration
The elements of Group16 have six electrons in the outermost shell and have ns2np4 general electronic configuration.
2. Atomic and Ionic Radii
Down the group atomic and ionic radii increase, due to increase in number of shells. Size of oxygen is exceptionally small.
3. Ionization Enthalpy
Ionization enthalpy decreases down the group, due to increase in size. However Group 16 have lower ionization enthalpy compared to Group 15 in a particular period, as Group 15 have extra stable half-filled p orbital electronic configuration.
4. Electron Gain Enthalpy
Because of the compact nature of oxygen atom, it has less negative electron gain enthalpy than sulphur.
5. Electronegativity
After fluorine, oxygen has the highest electronegativity. Down the group, electronegativity decreases.
6. Physical Properties
Oxygen and sulphur are non-metals, selenium and tellurium metalloids, whereas polonium is a metal. Polonium is radioactive and is short lived. All these elements exhibit allotropy. The melting and boiling points increase with an increase in atomic number down the group.
5. Chemical Properties
Oxidation states : Since electronegativity of oxygen is very high, it shows only negative oxidation state as –2 (except in OF2, where it is +2). Stability of –2 state decreases down the group. Polonium hardly shows –2 state. Other elements of the group show +2, +4, +6, where +4 and +6 are more common. S, Se, Te show +4 state with oxygen and +6 with fluorine. Bonding in +4 and +6 are primarily covalent.
Anomalous behaviour of oxygen
The anomalous behaviour of oxygen, like other members of p-block present in second period is due to its small size and high electronegativity. One typical example of effects of small size and high electronegativity is the presence of strong hydrogen bonding in H2O which is not found in H2S.
Dioxygen
Preparation
By heating oxygen containing salts such as chlorates, nitrates and permanganates.
2KClO3 ⟶ 2KCl + 3O2
Hydrogen peroxide is readily decomposed into water and dioxygen by catalysts such as finely divided metals and manganese dioxide.
2H2O2(aq) ⟶ 2H2O(l) + O2(g)
Properties
- O2 is colourless and odourless gas.
- Solubility in water is approx. 3.08 cm3 in 100 cm3 water at 293 K, sufficient for marine and aquatic life.
- It liquefies at 90 K and freezes at 55 K.
- It has three stable isotopes : O16, O17 and O18.
- O2 is paramagnetic and this can be explained on basis of MO theory.
Simple Oxides
Simple oxides can be classified as :
(i) Acidic Oxide
Non metal oxides are acidic but oxides of some metals in higher oxidation state also show acidic character. They combine with water to give acid e.g., SO2, Cl2O7, CO2, N2O5
For example, SO2 combines with water to give sulphurous acid.
SO2 + H2O ⟶ H2SO3
(ii) Basic Oxide
In general metallic oxides are basic. These oxides give base with water e.g., Na2O, CaO, BaO etc.
For example, CaO combines with water to given Ca(OH)2, a base.
CaO + H2O ⟶ Ca(OH)2
(iii) Amphoteric Oxide
Some metallic oxides show dual behaviour of both acid as well as alkali e.g., Al2O3
Al2O3(s) + 6HCl(aq) + 9H2O(l) ⟶ 2[Al(H2O)6]+3(aq) 6Cl-(aq)
Al2O3(s) + 6NaOH + 3H2O(l) ⟶ 2Na3[Al(OH)6](aq)
(iv) Neutral oxides
They are neither acidic nor basic e.g., CO, NO and N2O
(v) Mixed oxides
Oxides containing more than one cations with different oxidation states are called mixed oxides. e.g., Pb3O4, MgAl2O4, Fe3O4 etc.
Ozone
It is allotropic form of oxygen. Being too reactive it cannot remain in atmosphere at sea level. At height of 20 km, it is formed from atmospheric oxygen in the presence of sunlight. Ozone layer prevents earth’s surface from excessive exposure of UV radiations.
Preparation
On passing dry stream of O2 through silent electrical discharge, so as to prevent decomposition of O3. The product is known as ozonized oxygen.
3O2 ⟶ 2O3
Structure
It has angular structure and two oxygen-oxygen bond length in ozone are identical (128 pm) and bond angle 117º.
Properties
- O3 is pale blue gas, dark blue liquid and violet black solid.
- It has characteristic smell and in small concentration it is harmless. But in concentration above 100 ppm breathing becomes uncomfortable resulting in headache and nausea.
- O3 is thermodynamically unstable with respect to oxygen, as its decomposition liberates heat. Its conversion to O2 is favourable as ΔG is negative.
- Due to ease to release nascent oxygen O3 acts as strong oxidising agent, [O3 ⟶ O2 + O]
Uses
It is used as a germicide, disinfectant and for sterilising water. It is also used for bleaching oils, ivory, flour, starch, etc. It acts as an oxidising agent in the manufacture of potassium permanganate.
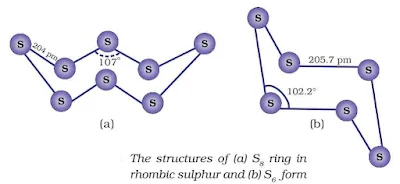
Allotropes of Sulphur
Rhombic sulphur (α-sulphur)
This allotrope is yellow in colour, m.p. 385.8 K and specific gravity 2.06. Rhombic sulphur crystals are formed on evaporating the solution of roll sulphur in CS2. It is insoluble in water but dissolves to some extent in benzene, alcohol and ether. It is readily soluble in CS2.
Monoclinic sulphur (β-sulphur)
Its m.p. is 393 K and specific gravity 1.98. It is soluble in CS2. This form of sulphur is prepared by melting rhombic sulphur in a dish and cooling, till crust is formed. On removing the crust, colourless needle shaped crystals of β-sulphur are formed. It is stable above 369 K and transforms into α-sulphur below it.
Compounds of Sulphur
(i). Sulphur Dioxide
Preparation
Sulphur dioxide is formed together with a little (6-8%) sulphur trioxide when sulphur is burnt in air or oxygen:
S(s) + O2(g) ⟶ SO2(g)
Industrially, it is produced as a by-product of the roasting of sulphide ores.
4FeS2 + 11O2 ⟶ 2Fe2O3 + 8SO2
Structure
Properties
Sulphur dioxide is a colourless gas with pungent smell and is highly soluble in water. It liquefies at room temperature under a pressure of two atmospheres and boils at 263 K.
Sulphur dioxide, when passed through water, forms a solution of sulphurous acid.
SO2 + H2O ⟶ H2SO3
(ii). Sulphuric Acid
Preparation
Sulphuric acid is manufactured by the Contact Process which involves three steps:
- burning of sulphur or sulphide ores in air to generate SO2.
- conversion of SO2 to SO3 by the reaction with oxygen in the presence of a catalyst (V2O5), and
- absorption of SO3 in H2SO4 to give Oleum (H2S2O7).
The key step in the manufacture of H2SO4 is the catalytic oxidation of SO2 with O2 to give SO3 in the presence of V2O5 (catalyst).
2SO2 + O2 ⟶ 2SO3
Lastly, SO3 obtained is dissolved in 98% H2SO4 when oleum is formed. H2SO4 of any desired concentration can be obtained from oleum by dilution with water.
H2SO4 + SO3 ⟶ H2S2O7
H2SO4 + H2O ⟶ 2H2SO4
Properties
It is colourless, syrupy oily liquid with corrosive nature. It's high B.P and viscous nature is due to H-bonding. It fumes strongly in moist air and soluble in water with evolution of heat due to formation of hydrates.
NaOH + H2SO4 ⟶ NaHSO4 + H2O
H2SO4 ⟶ H2O + SO2 + O
Uses
It is needed for the manufacture of hundreds of other compounds and also in many industrial processes. The bulk of sulphuric acid produced is used in the manufacture of fertilisers (e.g., ammonium sulphate, superphosphate). Other uses are in:
- (a) petroleum refining
- (b) manufacture of pigments, paints and dyestuff intermediates
- (c) detergent industry
- (d) metallurgical applications (e.g., cleansing metals before enameling, electroplating and galvanising)
- (e) storage batteries
- (f) in the manufacture of nitrocellulose products and
- (g) as a laboratory reagent.
Group 17 Elements
Fluorine, chlorine, bromine, iodine, astatine and tennessine are members of Group 17. These are collectively known as the halogens. The halogens are highly reactive non-metallic elements. Like Groups 1 and 2, the elements of Group 17 show great similarity amongst themselves. Astatine and tennessine are radioactive elements.
Properties of Group 16
1. Electronic Configuration
All these elements have seven electrons in their outermost shell (ns2np5) which is one electron short of the next noble gas.
2. Atomic and Ionic Radii
In a respective period, halogens have smallest atomic radii due to maximum effective nuclear charge. Down the group from F to I, atomic and ionic radii increases.
3. Ionisation Enthalpy
They have high ionization enthalpy, thus have little tendency to lose electron. Down the group ionization enthalpy decreases due to increase in size.
4. Electron Gain Enthalpy
Halogens have maximum negative ΔegH in corresponding period, as they are just short of one electron from stable noble gas configuration. Down the group ΔegH becomes less negative.
5. Electronegativity
Halogens have high electronegativity. Fluorine is the most electronegative element in the periodic table. Down the group electronegativity decreases.
6. Physical Properties
Fluorine and chlorine are gases, bromine is a liquid and iodine is a solid. Their melting and boiling points steadily increase with atomic number. All halogens are coloured. Fluorine and chlorine react with water. Bromine and iodine are only sparingly soluble in water but are soluble in various organic solvents such as chloroform, carbon tetrachloride, carbon disulphide and hydrocarbons to give coloured solutions.
7. Chemical Properties
Oxidation states : All the halogens exhibit –1 oxidation state. However, chlorine, bromine and iodine exhibit +1, +3, +5 and +7 oxidation states.
Chemical Reactivity : All halogens are highly reactive. They react with metals and non-metals to form halides. Reactivity of halogen decreases down the group.
Anomalous behaviour of fluorine
The anomalous behaviour of fluorine is due to its small size, highest electronegativity, low F-F bond dissociation enthalpy, and non availability of d orbitals in valence shell. ionisation enthalpy, electronegativity, and electrode potentials are all higher for fluorine than expected from the trends set by other halogens. Also, ionic and covalent radii, m.p. and b.p., enthalpy of bond dissociation and electron gain enthalpy are quite lower than expected.
Chlorine
Chlorine was discovered in 1774 by Scheele by the action of HCl on MnO2. In 1810 Davy established its elementary nature and suggested the name chlorine on account of its colour.
Preparation
It can be prepared by heating manganese dioxide with concentrated hydrochloric acid.
MnO2 + 4HCl ⟶ MnCl2 + Cl2 + 2H2O
Manufacture of chlorine
Deacon’s process: By oxidation of hydrogen chloride gas by atmospheric oxygen in the presence of CuCl2 (catalyst) at 723 K.
4HCl + O2 ⟶ 2Cl2 + 2H2O
Properties
It is a greenish yellow gas with pungent and suffocating odour. It is about 2-5 times heavier than air. It can be liquefied easily into greenish yellow liquid which boils at 239 K. It is soluble in water.
It reacts with compounds containing hydrogen to form HCl.
H2 + Cl2 ⟶ 2HCl
H2S + Cl2 ⟶ 2HCl + S
Uses
- It is used for bleaching woodpulp, bleaching cotton and textiles.
- It is used in the extraction of gold and platinum.
- It is used in the manufacture of dyes, drugs and organic compounds such as CCl4, CHCl3, DDT, refrigerants, etc.
- It is used in sterilising drinking water.
- It is used in preparation of poisonous gases such as phosgene (COCl2), tear gas (CCl3NO2), mustard gas (ClCH2CH2SCH2CH2Cl).
Hydrogen Chlorid
Glauber prepared this acid in 1648 by heating common salt with concentrated sulphuric acid.
Preparation
In laboratory, it is prepared by heating sodium chloride with concentrated sulphuric acid.
NaCl + H2SO4 ⟶ NaHSO4 + HCl
Properties
It is a colourless and pungent smelling gas. It is easily liquefied to a colourless liquid (b.p.189 K) and freezes to a white crystalline solid (f.p. 159 K). It is extremely soluble in water and ionises as follows:
HCl(g) + H2O ⟶ H3O+ + Cl-
Its aqueous solution is called hydrochloric acid. High value of dissociation constant (Ka) indicates that it is a strong acid in water.
When three parts of concentrated HCl and one part of concentrated HNO3 are mixed, aqua regia is formed which is used for dissolving noble metals, e.g., gold, platinum.
Uses
- It is used in the manufacture of chlorine, NH4Cl and glucose.
- It is used for extracting glue from bones and purifying bone black.
- It is used in medicine and as a laboratory reagent.
Oxoacids of Halogens
Due to high electronegativity and small size, fluorine forms only one oxoacid, HOF known as Fluoric (I) acid or hypofluorous acid. Other halogens form number of oxoacids, most of them cannot be isolated in pure state. They are stable only in aqueous solution or in form of their salts.
Interhalogen Compounds
When two different halogens react with each other, interhalogen compounds are formed. They can be assigned general compositions as XX′, XX′3, XX′5 and XX′7 where X is halogen of larger size and X′ of smaller size and X is more electropositive than X′.
Preparation
The interhalogen compounds can be prepared by the direct combination or by the action of halogen on lower interhalogen compounds. The product formed depends upon some specific
conditions.Cl2 + F2 ⟶ 2ClF
Properties
These are all covalent molecules and are diamagnetic in nature. They are volatile solids or liquids at 298 K except ClF which is a gas. Their physical properties are intermediate between those of constituent halogens except that their m.p. and b.p. are a little higher than expected.
Uses
These compounds can be used as non aqueous solvents. Interhalogen compounds are very useful fluorinating agents. ClF3 and BrF3 are used for the production of UF6 in the enrichment of 235U.
Group 18 Elements
Group 18 consists of elements: helium, neon, argon, krypton, xenon, radon and oganesson. All these are gases and chemically unreactive. They form very few compounds, because of this they are termed as noble gases.
Electronic Configuration
All noble gases have general electronic configuration ns2np6 except helium which has 1s2.
Ionisation Enthalpy
Due to stable electronic configuration, these gases have very high ionization enthalpy. Down the group it decreases with increase in atomic size.
Atomic Radii
It increases down the group with increase in atomic number.
Electron Gain Enthalpy
As noble gases have stable electronic configuration, they have no tendency to accept electron and therefore large positive value of electron gain enthalpy.
Physical Properties
- They are monoatomic, colourless, odourless and tasteless.
- Sparingly soluble in water.
- Very low melting and boiling points as weak dispersion forces present.
- Helium has lowest boiling point (4.2 K) of any known substance.
- It has unusual property of diffusing through commonly used laboratory materials like rubber, glass or plastics.
- Noble gases are least reactive. Their chemical inertness is due to Completely filled valence shell.
Chemical Properties
Xe(g) + F2(g) ⟶ XeF2(s)
Xe(g) + 2F2(g) ⟶ XeF4(s)
Xe(g) + 3F2(g) ⟶ XeF6(s)
XeF6 + 3H2O ⟶ XeO3 + 6HF
Summary
Inert Pair Effect: The inability of valence shell ns2 electrons to take part in bond formation in p-block is known as inert pair effect.
Disproportionation: Same element gets oxidised as well as reduced in a chemical reaction.
pπ-pπ bond: The chemical bond (π) formed by lateral (sidewise) overlapping of p orbitals of two atoms in a compound or ion.
dπ-pπ bond: The chemical bond (π) formed by lateral overlapping of p and d orbitals of two atoms in a compound or ion.
Allotropy: Phenomenon of existence of an element in different physical forms.
Catenation: Tendency of self linking of element.
Chalcogens: Ore forming. Given to Group 16.
Halogens: Salt producers. Given to Group 17.
Interhalogen compound: Compounds formed between two different halgoen.
Noble gases: Elements of Group 18, as they form very few compounds that too under certain condition.
Anomalous behaviour: The unique behaviour of 1st member of a particular group from other members of same group.

















Join the conversation